 Loading...
Loading... Loading...
Loading...August 1, 2023
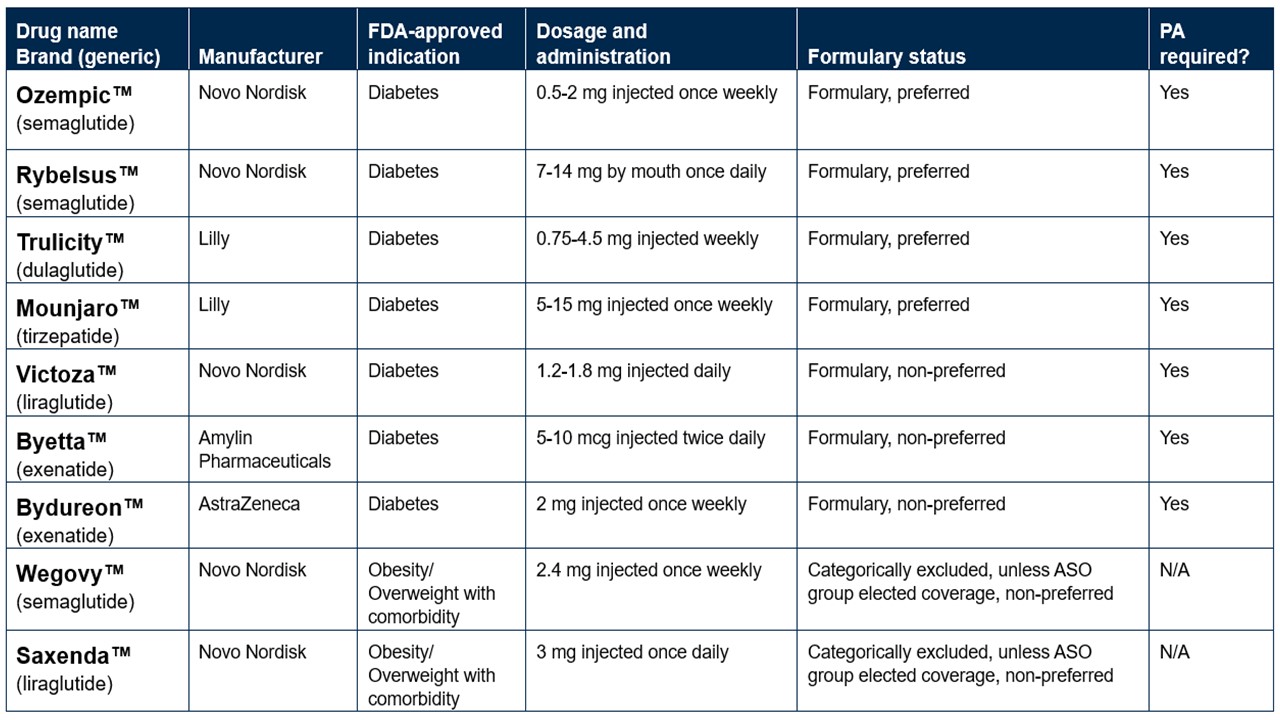
The rapid increase in use of GLP-1 (glucagon-like peptide-1) drugs, such as Ozempic, Rybelsus and Trulicity, in 2023 has required us to take action to help manage employer groups’ financial risk.
GLP-1 drugs have been proven to be highly effective in the treatment of type 2 diabetes. In December 2022, the American Diabetes Association included GLP-1 use in its standard of care for treatment of diabetes. GLP-1 drugs have also been shown to be effective in treating obesity. However, our benefits exclude coverage for obesity medications.
To ensure these drugs are covered based on our benefit design, we'll implement a pre-authorization (PA) requirement on Oct. 1, 2023, to ensure use for diabetes only.
Members with indication of type 2 diabetes based on medical claims over the past 24 months and those who’ve been prescribed drugs for the treatment of type 2 diabetes over the past 120 days will be exempt from PA and continue to receive coverage for GLP-1s. This represents the majority of members who are currently using GLP-1 products. We won't communicate PA requirements to these members.
We will communicate next steps to the estimated 30 to 35% of members whose continued coverage after Oct. 1 will require PA.
GLP-1 drugs FDA approved for treating an obesity diagnosis (Wegovy and Saxenda) are excluded from coverage for all lines of business. Self-funded groups may customize.
Below are some of the more common GLP-1 drugs, their FDA-labeled indications and how they’re placed on the formulary as of Oct. 1, 2023.
On July 1, 2023, we added Humira biosimilars Hadlima and Amjevita to our formulary, while continuing to include Humira as a choice.
We moved quickly to evaluate and select Humira biosimilars for our formulary based on safety, efficacy and value. Many providers are reluctant to switch patients who are doing well on their current medication. Keeping Humira on our formulary will minimize disruption to therapy for complex conditions while ensuring that doctors and patients have choice and flexibility.
If you have any questions about these changes, please contact your account representative.